
1.1Risk assessment for Torsades de pointes (TdP) caused by delayed repolarization
We perform risk assessment to predict Torsades de pointes (Tdp) caused by delayed repolarization by measuring extracellular field potential using human iPS cell-derived cardiomyocytes (hiPSC-CMs) with MEA (multi-electrode array) under rigorously controlled temperature, humidity, and CO2 concentration.
LSIM is the only contract laboratory in Japan which has offered field potential measurement data using hiPSC-CMs to an international validation test (CiPA initiative) lead by HESI/FDA (http://cipaproject.org/).
1.2Performance evaluation of hiPSC-CMs and measuring instruments
Since joining the national project (NEDO) from 2009 the first of all CROs in Japan, LSIM has become the leader in system development of proarrhythmia prediction utilizing hiPSC-CMs. From this experience and earned expertise, LSIM is able to accurately grasp the clients' needs for hiPSC-CMs and measuring instruments, and provide appropriate studies and services for product development.
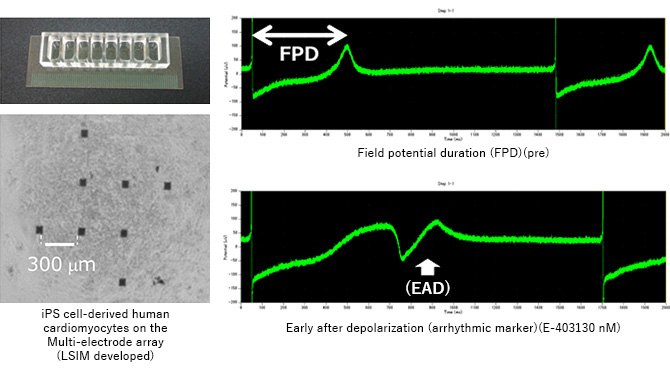
LSIM Safety Institute submitted Field-potential data from human iPS cardiomyocytes to the CiPA initiative*1. We offer the testing as GLP-compliant service for regulatory use according to the ICH S7B, as well as for early drug development.
*1; Blinova K., et al., Cell Report 24, 3582-3592, 2018