
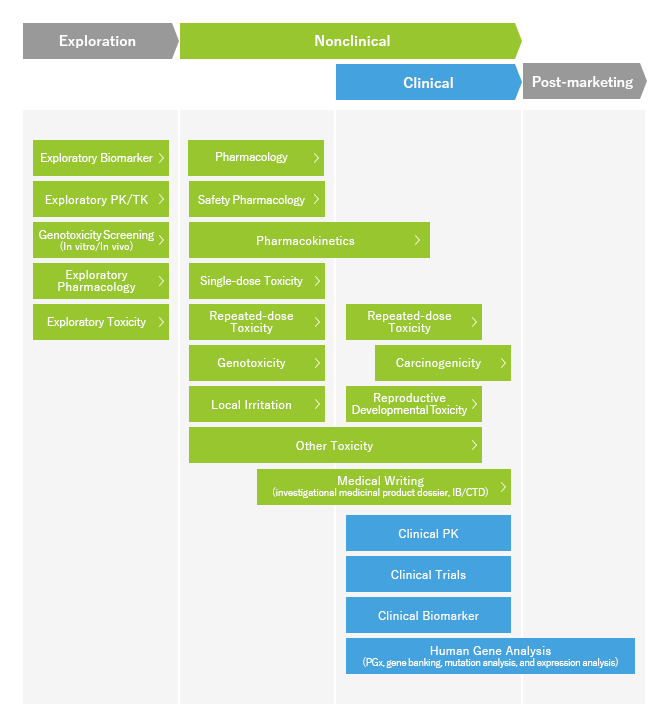
LSIM Safety Institute supports development of pharmaceutical products by providing a series of nonclinical services from the early stages of drug development to application for manufacturing and marketing approval. In addition to possessing a wide range of efficacy models in pharmacological evaluation, we are also able to offer development of models according to our clients�f needs. For toxicity and safety pharmacology studies, various safety tests necessary for application of manufacturing and marketing approval are conducted under pharmaceutical GLP conditions in compliance with ICH guidelines. We can also comprehensively address Pharmacokinetic studies including exploratory tests. Our staff members have extensive experience and skills in their areas of expertise and will provide high quality and reliable results in all studies under GLP and Reliability Criteria.
If within one project there are multiple studies, a project manager will serve as a point of contact for the client and through smooth communication present an efficient study protocol, accurately conduct progress management and reporting on the project, and present comprehensive evaluation results consistent among studies.
LSIM Safety Institute provides medical writing services to support application for regulatory approval. We produce high quality documents which include investor�fs brochure (IB), non-clinical sections of IB and CTC with QC/QA verification.

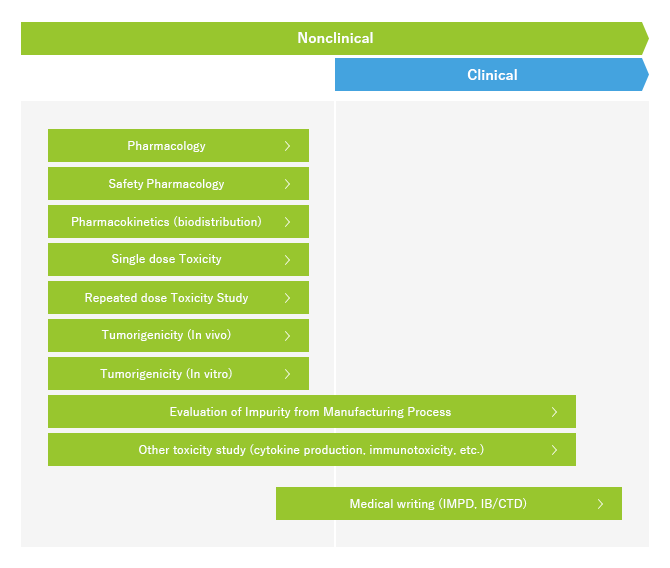
At LSIM Safety Institute, a system to accept nonclinical safety studies of regenerative medicine products has been developed. We are certified with GLP for regenerative medicine products and we conduct toxicity studies necessary for application for manufacturing and marketing approval in accordance with GLP and related guidelines. In regards to pharmacological studies, we have disease models in various categories such as hepatitis, peripheral artery disease, arthritis, myocardial infarction, and cerebral infraction, and also develop models according to our clients�f requests. We are able to offer biodistribution studies for pharmacokinetics. Our staff has extensive experience and expertise, and will provide reliable results in all studies including GLP and criteria for reliability.
If multiple studies are consigned for one project, the project manager will act as a point of contact with the client, present an efficient study plan for the project, manage accurate progress and adequately report through smooth communication while also presenting comprehensive evaluation results consistent between the studies.
We are also entrusted with medical writing work as medicinal application support. We provide high quality documents with QC/QA verification which are non-clinical sections of investigational medicinal product dossiers (IMPD), investigator's brochures (IB), and common technical documents (CTD).

| In vivo | Animal species: General animal and immunodeficient animal (nude mouse/NOG mouse/nude rat/others) |
|---|---|
| Single dose toxicity study | |
| Repeated dose toxicity study | |
| Tumorigenicity study | |
| In vitro | Soft agar colony formation assay |
| Hepatopathy model | Non-alcoholic steatohepatitis (NASH) |
|---|---|
| Carbon tetrachloride-induced hepatitis (acute/chronic) | |
| Concanavalin A (ConA)-induced hepatopathy | |
| Thioacetamide (TAA)-induced liver fibrosis | |
| Peripheral arterial disease (PAD) model | Hind limb ischemia |
| Arthritis model | Type �U Collagen-induced arthritis Osteoarthritis |
| Myocardial infraction model | Ischemic reperfusion, permanent occlusion |
| Cerebral ischemia model | Transient focal cerebral ischemia |
| Nephritis model | Puromycin-induced nephritis model (rat) |
In addition, please contact us about the possibility of conducting other studies using the test items of pharmacological study.
LSIM Safety Institute has developed a system for conducting non-clinical studies of medical devices. Since being confirmed to comply with medical device GLP in safety studies (May 28, 2018) , we have conducted toxicity studies in full compliance with GLP and/or other related guidelines necessary for applications of manufacturing and marketing approval. Our personnel have extensive experience and skills in their areas of responsibility and are able to provide high quality and reliable results in all studies that include GLP and reliability criteria.
If within one project there are multiple studies, a project manager will serve as a point of contact for the client and through smooth communication present an efficient study protocol, accurately conduct progress management and reporting on the project, and present comprehensive evaluation results consistent among studies.
LSIM Safety Institute provides medical writing services to support application for regulatory approval. We produce high quality documents with QC/QA verification which are non-clinical sections of investigational medicinal product dossiers (IMPD), investigator's brochures (IB), and common technical documents (CTD).
LSIM Safety Institute conducts various toxicity studies in compliance with GLP for agricultural chemicals and OECD guidelines necessary for application for pesticide registration.
LSIM Safety Institute provides reliable data through various safety studies that are required for approval of specified health supplementary foods and food additives, etc. We can conduct these studies in compliance with pharmaceutical GLP.
LSIM Safety Institute provides highly reliable data required for application for marketing approval of quasi drugs, etc., through various toxicity studies listed below. We can conduct these studies in compliance with pharmaceutical GLP.
LSIM Safety Institute provides highly reliable data required for application for approval of veterinary drugs through various toxicity studies listed below. We can conduct these studies in compliance with GLP for veterinary drugs.
LSIM Safety Institute conducts the following studies related to safety assessment of chemical substances.
In response to our client's request, we offer a wide range of services from simple study to various safety studies which apply to Japanese Chemical Substances Control Law (CSCL) or Industrial Safety and Health Law GLP.